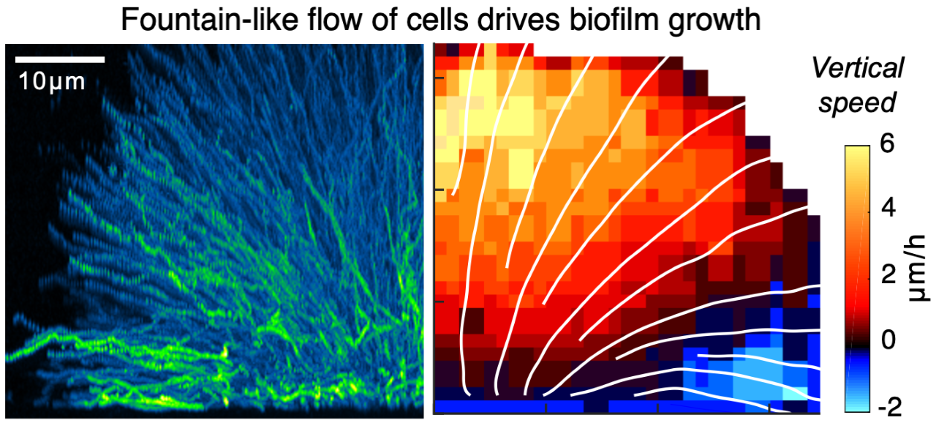
We developed dual-view light-sheet microscopy to investigate the dynamics of biofilm development from a founder cell to a mature three-dimensional community. Tracking of individual cells revealed two distinct fates: one set of biofilm cells expanded ballistically outward, while the other became trapped at the substrate. A collective fountain-like flow transported cells to the biofilm front, bypassing members trapped at the substrate and facilitating lateral biofilm expansion. This collective flow pattern was quantitatively captured by a continuum model of biofilm growth against substrate friction. Coordinated cell movement required the matrix protein RbmA, without which cells expanded erratically. Thus, tracking cell lineages and trajectories in space and time revealed how multicellular structures form from a single founder cell. The collective cellular flow patterns and cell-matrix substrate interaction principles may be relevant in other prokaryotic and eukaryotic systems and could underpin multicellular development and organization.

