Biofilms are ubiquitous surface-attached bacterial communities embedded in an extracellular matrix. Quorum sensing controls biofilm formation. We demonstrated that flow through soil-like porous materials, industrial filters, and medical stents dramatically modifies the morphology of biofilms to form streamers, which, over time, bridge the spaces between obstacles in non-uniform environments. We developed microfluidic systems to show that, contrary to the current paradigm, accumulation of surface-attached biofilms has little effect on flow, whereas biofilm streamers cause sudden and rapid clogging. We showed that quorum sensing controls streamer formation and the quorum-sensing antagonists that we discovered inhibit streamer formation
Some factors secreted from biofilms behave as public goods because they can be exploited by non-producing cells (e.g., extracellular enzymes that solubilize solid substrates). The means by which public-goods-producing bacteria avert exploitation in biofilm environments has been mysterious. We showed that the public goods dilemma may be solved by two different mechanisms: cells can produce thick biofilms that confine goods to producers, and/or fluid flow can remove soluble products of digestion, denying access to non-producers. Both processes limit the distance over which enzyme-secreting cells provide benefits to neighbors, resulting in preferential benefit to nearby clonemates and allowing kin selection to favor public goods production. We next investigated defense against biofilm invasion. We showed that immotile cells, including those identical to the biofilm resident strain, are completely excluded from entry into resident biofilms. Motile cells can transiently colonize and grow on the biofilm exterior, but are readily removed by shear forces. We found that, in vibrios, protection from invasion is mediated by the secreted protein RbmA, which binds mother-daughter cell pairs together and to the matrix. RbmA is not shared, nor is the invasion protection it confers. Indeed, we showed that RbmA strictly localizes to the producing cell lineages.
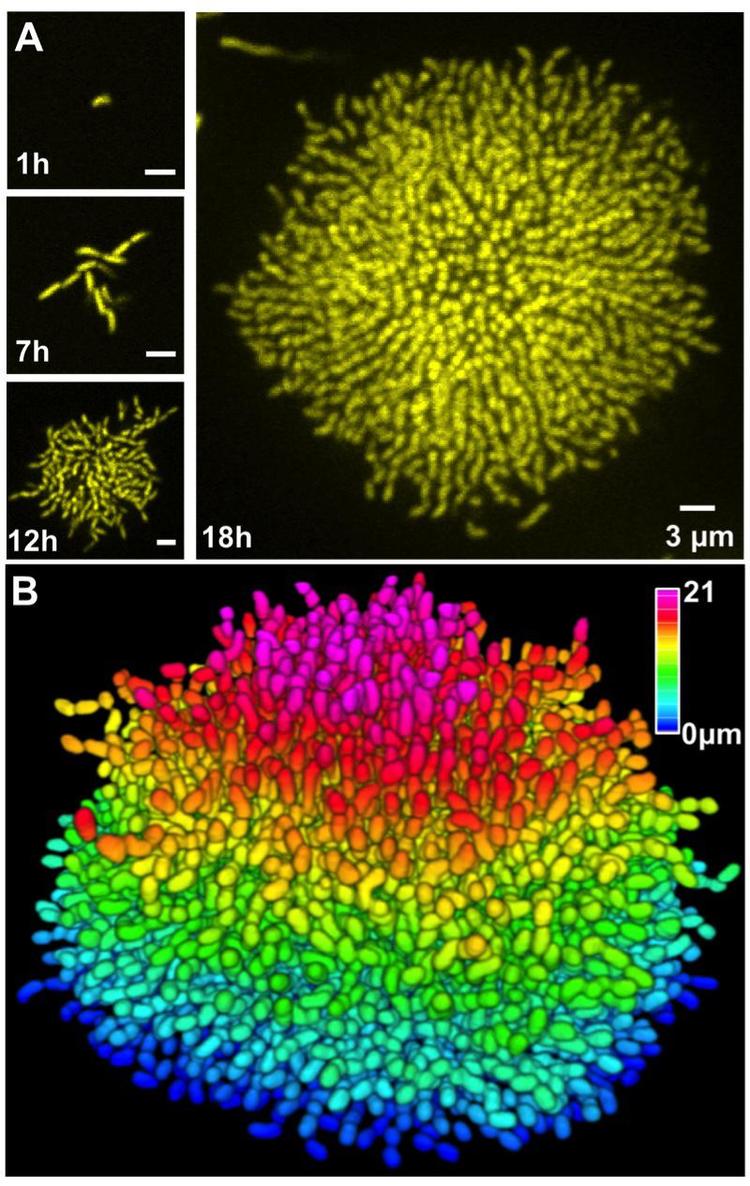
Furthermore, despite thousands of publications on biofilms, we know only a few key facts, e.g. matrix production and quorum sensing are required for biofilm formation. We have little understanding of how cells are arranged within a biofilm, which genes are key in different spatial and temporal domains, which cells are dividing, how nutrient and signal molecules transit the structure, etc. Past investigations have been limited to optical studies of biofilm formation with only a few cells or to gross characterization of the entire structure. We recently made an imaging breakthrough critical for the proposed work: we built a microscope capable of resolving and tracking individual cells in living, growing biofilms from the founder cell to 10,000 cells. To our knowledge, this is the first time anyone has peered into a biofilm to watch it develop, cell by cell. Our studies are done in the presence of flow, under topographical conditions mimicking environmental, medical, and industrial systems. Broadly speaking, we will define the “structure-function” relationships connecting the microscale structure of biofilms and their corresponding gene expression patterns with the macroscopic physical properties of biofilms, e.g. viscoelasticity, structural strength, resistance to invading cells, and impermeability to antibiotics.

