Many bacteria harness information garnered from multiple autoinducer receptors and pathways to regulate behavior. We think that integrating information encoded in autoinducer mixtures facilitates intra-species, intra-genus, and inter-species communication, enabling bacteria to precisely execute niche-specific behaviors. Dissecting autoinducer-receptor interactions could enable an understanding of how organisms decode information-containing molecules and inspire strategies to manipulate both harmful and beneficial quorum-sensing-controlled processes.
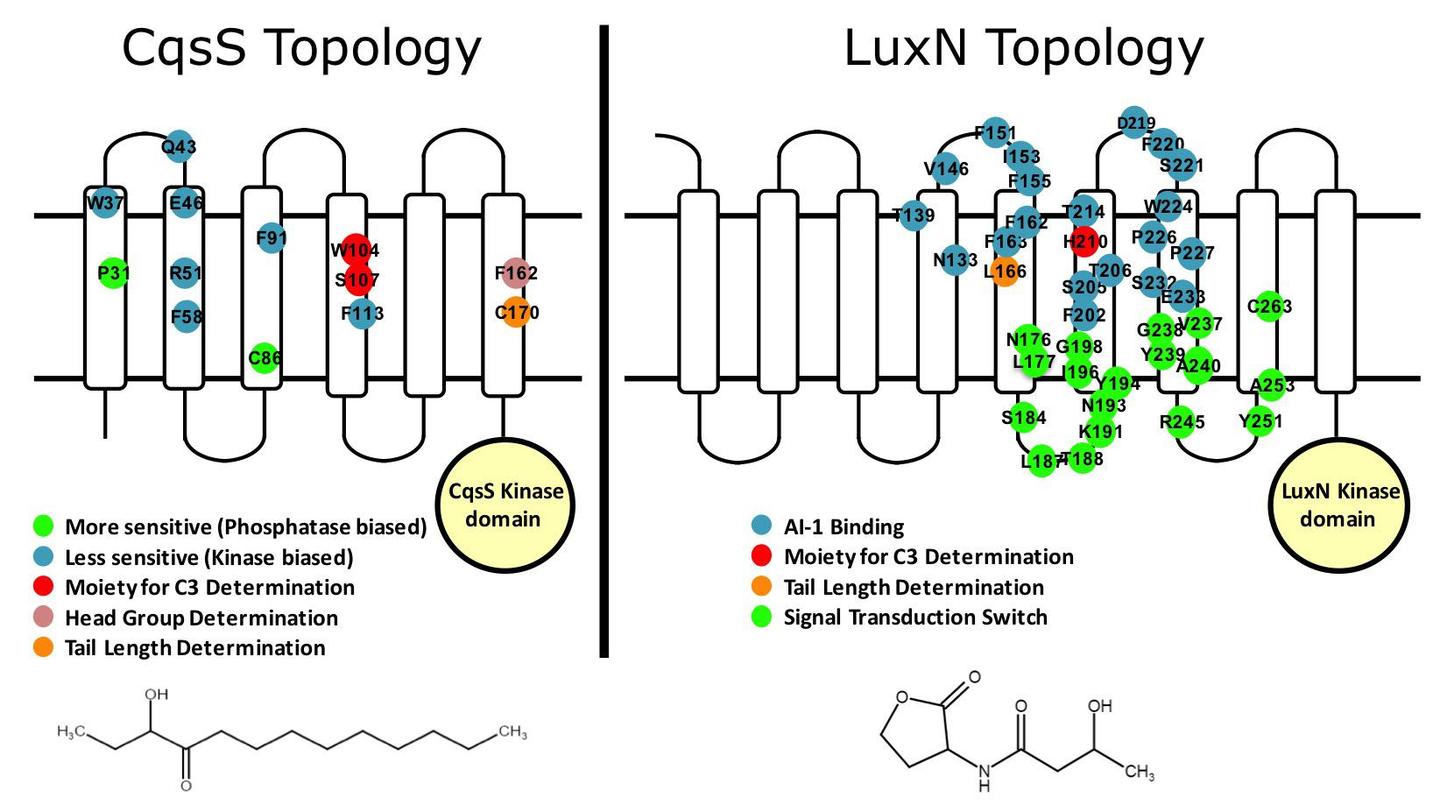
To understand signal discrimination, we carried out screens to identify agonists and antagonists of the V. harveyi LuxN and V. cholerae CqsS autoinducer receptors that detect AI-1 (3OH-C4 homoserine lactone) and CAI-1 (3-aminotridecan-4-one), respectively. We modified the ligands by synthetic chemistry and the receptors by mutagenesis to identify the receptors’ autoinducer-binding domains and mutants with altered autoinducer sensitivity/ligand recognition profiles. We assigned specific receptor amino acids to particular roles in recognition of specific moieties in the native ligands and ligand analogs. In the case of LuxN, we also identified the region acting as the switch to trigger signal relay. Our analyses illuminated how these two quorum-sensing receptors distinguish between highly similar ligands and exploit those differences to regulate signaling activity. Furthermore, this work demonstrated that we can make molecules that precisely control the activity of two-component receptors – a large and highly studied family of bacterial receptors.
Two-component sensory systems often respond to complex environmental information such as osmolarity or stress, and thus the molecular nature of the signals is poorly understood. Because quorum-sensing systems rely on known molecules as ligands, we can exploit them to answer long-standing questions about how ligand binding and response occur.
The V. harveyi LuxN ligand is a homoserine lactone with a C4 tail. Our studies showed that longer tailed analogs are potent antagonists in the order C12>C10>C8>C6. We suggest that, in its native environment, V. harveyi encounters mixtures of signals produced by other species occupying the same niche. Possibly, V. harveyi uses relative antagonism potency to measure self, non-self, and how closely or distantly related it is to other species. Rather than “ignoring” longer signal molecules by not binding them, we propose that receptor antagonism prevents leakage of benefits of quorum-sensing-controlled public goods to non-kin.