With respect to the microbiota: we recently discovered a new quorum-sensing system in V. cholerae that relies on an extracellular autoinducer, a cytoplasmic autoinducer receptor (VqmA), and a downstream regulatory sRNA (VqmR) that controls 17 target genes including those required for biofilm formation. We used fractionation, mass spectroscopy, NMR, and synthesis to identify the new autoinducer as 3,5-dimethylpyrazin-2-ol. During V. cholerae infection, the autoinducer is generated by the host microbiota by digestion of the mucin lining the intestine. Our data show that release of the mucin-derived autoinducer represses V. cholerae biofilm formation, promoting expulsion from the host. Animal experiments will explore the possibility of using the new autoinducer in the treatment of cholera disease.
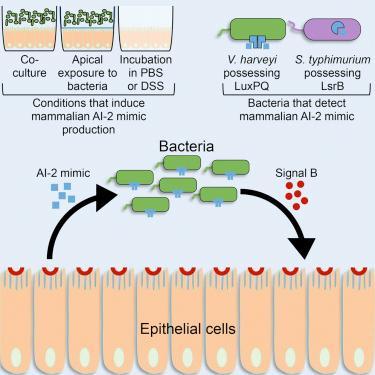
With respect to the host: We recently found that mammalian epithelial cells and host tissue of epithelial origin produce a mimic of the autoinducer AI-2. AI-2 is the universal inter-species autoinducer we discovered a decade ago. AI-2 mimic production is induced in host cells only after exposure to bacteria. Exploiting AI-2, as opposed to a highly species-specific autoinducer, could be a strategy that enables the host to maximally manipulate bacterial behavior in mixed populations such as those that exist in the gut. We are identifying the structure of the mimic using NMR, metabolomics, and synthesis. We will define the gene(s) required to make the mimic using CRISPR mutant libraries. We will determine what the mimic does to host cells using RNA-seq. We will do likewise with the bacteria, with the expectation that the mimic controls quorum-sensing-regulated behaviors. We will determine what happens to the microbiota and the host when the mimic is absent, present, or in excess. We will determine whether the mimic has roles in microbiota homeostasis and/or infection. These studies could provide a path to the discovery of other host-produced quorum-sensing molecules as more than one molecule is likely involved.