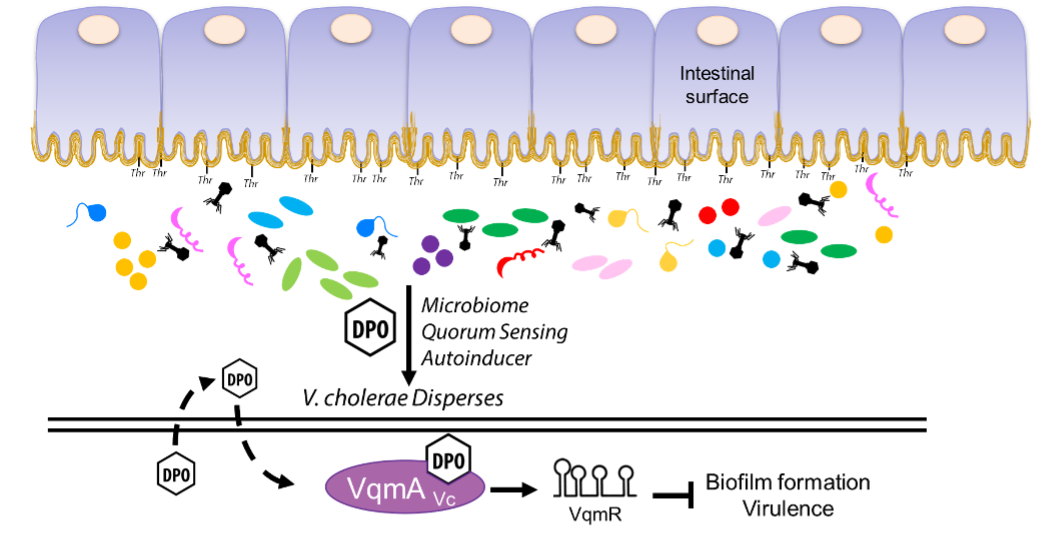
We recently discovered a new quorum-sensing system in Vibrio cholerae that relies on the cytoplasmic autoinducer receptor VqmA (VqmAVc) and the downstream regulatory small RNA VqmR. Using fractionation, mass spectroscopy, NMR, and synthesis, we identified the autoinducer in this system to be 3,5-dimethylpyrazin-2-ol (DPO).The discovery of DPO allowed us to complete the V. cholerae quorum-sensing circuit: upon binding DPO, VqmA activates transcription of a gene encoding a small RNA (sRNA), called VqmR, and VqmR represses genes involved in biofilm formation and virulence factor production. Repression of these traits is crucial for V. cholerae to disperse from its human host and disseminate to new victims. Commensal bacteria also make DPO and our experiments revealed that DPO is derived from threonine, the most abundant amino acid in the Muc2 protein that makes up human intestinal mucus that coats our intestinal cells. Our proposal is that the human host provides the substrate required by the microbiota bacteria to produce the autoinducer that defends us against an invading pathogen.
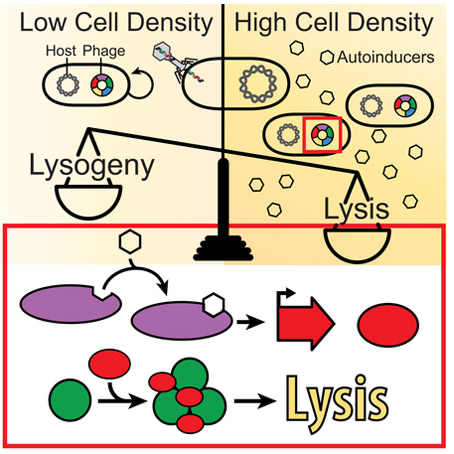
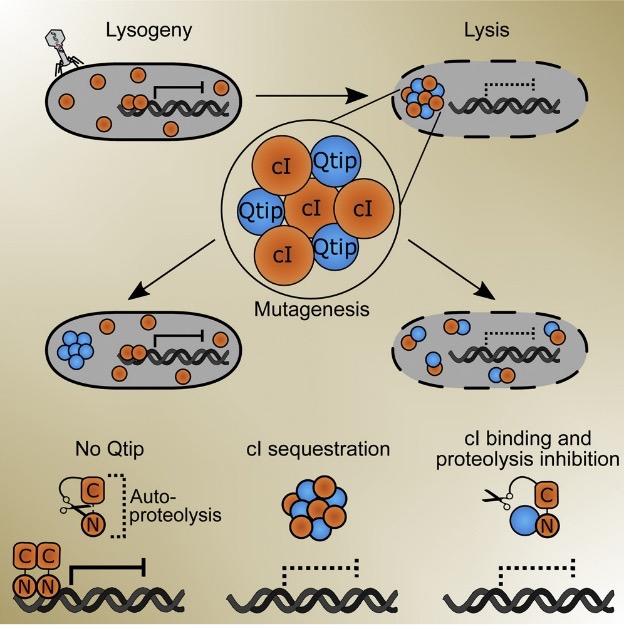
We also recently identified phage-encoded quorum-sensing systems. One such system is employed by the phage VP882, which infects and kills vibrios. Phage VP882 encodes a VqmA homolog, which we named VqmAPhage. Analogous to bacterial VqmA, VqmAPhage binds to DPO produced by its vibrio host. However, unlike bacterial quorum sensing, which benefits the bacterial population, activation of the VP882 phage-based quorum-sensing system promotes phage replication and killing of the bacterial host by triggering the production of a new phage-encoded protein we named Qtip. Qtip is a small protein with no predictable domains. To understand its function, we systematically mutagenized all amino acids in Qtip and pinpointed discrete residues that confer two different sets of properties both at the population-level, in terms of inducing lysis, and at the single-cell level, in terms of the intracellular localization of the Qtip protein. Beyond just studying existing phage-encoded quorum-sensing systems, we are using what we learn to design phages with re-engineered lysis-lysogeny modules under the control of synthetic inducers as well as temporal and optical cues that allow us to control phage behaviors in both space and time. In addition to their use as a research tool, we believe these phages could have broad uses in industrial, medical, and environmental applications.